
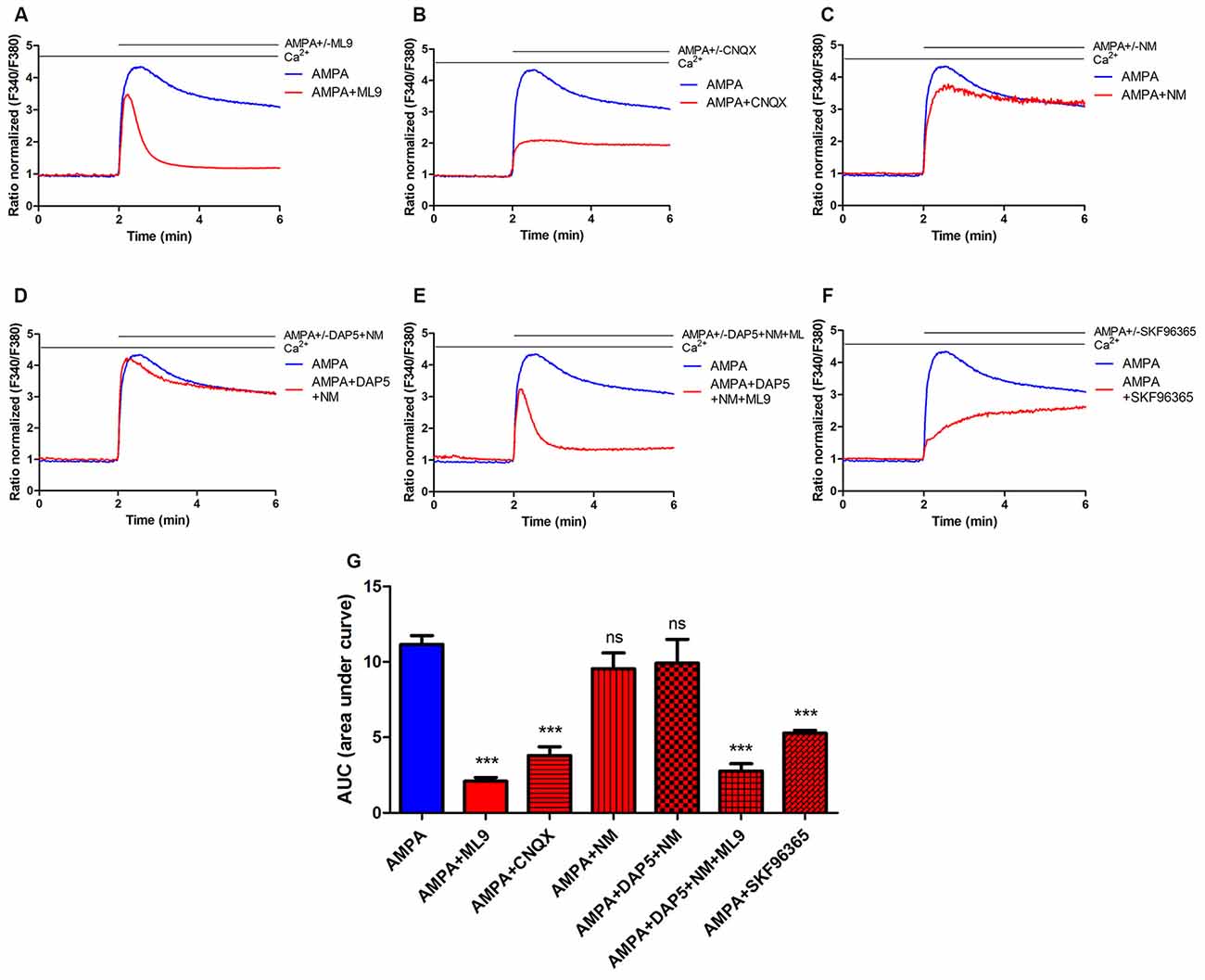

lMes neurons have more highly branched dendrites compared with mMes neurons, as indicated from the increased number of intersections. Data are shown as intersections of 1 μm eccentricities from the soma of 16 lMes and 18 mMes neurons. C, Three-dimensional Sholl analysis of reconstructed lMes and mMes neurons. Both neuronal types express TH and Ca v1.3, but only mMes neurons express Cb.
Calcium entry in neurons dendrite verification#
B, scRT-PCR verification of TH, Cb, and voltage-gated L-type Ca v1.3 calcium channels (Ca v1.3) mRNA expression in lMes and mMes neurons. Images show that mMes neurons express the calcium binding protein calbindin while lMes neurons do not.
Calcium entry in neurons dendrite serial#
A, Serial optical sections ( Z-stacks) were acquired on a confocal microscope of 2-week old lMes and mMes neurons from cocultures generated from mice expressing TH-GFP. Morphological differences between cultured lateral mesencephalon (lMes) and medial mesencephalon (mMes) neurons. These results show that physiological and proteostatic stress can be additive in the soma and dendrites of vulnerable dopaminergic neurons, providing new insight into the factors underlying PD pathogenesis. This stress appeared to be extramitochondrial in origin, because scavengers of cytosolic reactive oxygen species or inhibition of NADPH oxidase attenuated it. Moreover, the formation of intracellular α-synuclein Lewy-body-like aggregates increased mitochondrial oxidant stress in perinuclear and dendritic compartments. Examination of SNc dopaminergic neurons ex vivo in brain slices verified this pattern. This stress progressively increased with distance from the soma. Activity-dependent calcium entry through L-type channels increased mitochondrial oxidant stress in dendrites. Many of the key features of in vivo adult dopaminergic neurons were recapitulated in vitro. To pursue these questions, mesencephalic dopaminergic neurons derived from C57BL/6 transgenic mice were studied in primary cultures, allowing for visualization of soma and dendrites simultaneously. What is less clear is whether this physiological stress is present in dendrites and if Lewy bodies, the major neuropathological lesion found in PD brains, exacerbate it. Previous work has shown that activity-dependent calcium entry through L-type channels elevates perinuclear mitochondrial oxidant stress in SNc dopaminergic neurons, providing a potential basis for their selective vulnerability. Mitochondrial oxidant stress is widely viewed a major factor in PD pathogenesis. The core motor symptoms of Parkinson's disease (PD) are attributable to the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc).


 0 kommentar(er)
0 kommentar(er)
